Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yotsuji, Kenji*; Tachi, Yukio; Sakuma, Hiroshi*; Kawamura, Katsuyuki*
Applied Clay Science, 204, p.106034_1 - 106034_13, 2021/04
Times Cited Count:61 Percentile:99.73(Chemistry, Physical)Sugiura, Yuki; Ishidera, Takamitsu; Tachi, Yukio
Applied Clay Science, 200, p.105910_1 - 105910_10, 2021/01
Times Cited Count:6 Percentile:51.59(Chemistry, Physical)Okubo, Takahiro*; Yamazaki, Akio*; Fukatsu, Yuta; Tachi, Yukio
Microporous and Mesoporous Materials, 313, p.110841_1 - 110841_11, 2021/01
Times Cited Count:5 Percentile:29.65(Chemistry, Applied)Pore distributions in water-saturated Ca-montmorillonite were investigated using  H NMR measurements under various dry densities (0.8 - 1.6 g/cm
H NMR measurements under various dry densities (0.8 - 1.6 g/cm ) and porewater salinity conditions (deionized water, 0.1 and 1 M CaCl
) and porewater salinity conditions (deionized water, 0.1 and 1 M CaCl ), at the temperature range of 233 - 303 K. The volume fractions of the interlayer pore including two and three hydrated layers and the non-interlayer pore in compacted Ca-montmorillonite were quantified by NMR relaxometry including
), at the temperature range of 233 - 303 K. The volume fractions of the interlayer pore including two and three hydrated layers and the non-interlayer pore in compacted Ca-montmorillonite were quantified by NMR relaxometry including 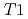 and
and 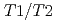 distribution analysis, and were compared with NMR cryoporometry and X-ray diffractometry. These analysis provided consistent pictures on the pore distributions in compacted Ca-montmorillonite, in contrast to Na-montmorillonite. The main factor affecting the pore distribution in compacted Ca- and Na-montmorillonite is the density, whereas the effect of porewater salinity is relatively smaller. The effect of interlayer cations is also relatively smaller at higher density, although the differences in the pore structures are significant at low density.
distribution analysis, and were compared with NMR cryoporometry and X-ray diffractometry. These analysis provided consistent pictures on the pore distributions in compacted Ca-montmorillonite, in contrast to Na-montmorillonite. The main factor affecting the pore distribution in compacted Ca- and Na-montmorillonite is the density, whereas the effect of porewater salinity is relatively smaller. The effect of interlayer cations is also relatively smaller at higher density, although the differences in the pore structures are significant at low density.
Sugiura, Yuki; Tomura, Tsutomu*; Ishidera, Takamitsu; Doi, Reisuke; Francisco, P. C. M.; Shiwaku, Hideaki; Kobayashi, Toru; Matsumura, Daiju; Takahashi, Yoshio*; Tachi, Yukio
Journal of Radioanalytical and Nuclear Chemistry, 324(2), p.615 - 622, 2020/05
Times Cited Count:4 Percentile:45.45(Chemistry, Analytical)Takahashi, Hiroaki*; Tachi, Yukio
Applied Clay Science, 168, p.211 - 222, 2019/02
Times Cited Count:9 Percentile:47.52(Chemistry, Physical)Microstructural and mass transport properties of compacted Na- and Cs-montmorillonites with different swelling properties were investigated by combining 3D microstructure analysis using nanofocus X-ray CT and diffusion measurement of HDO. The X-ray CT observations indicated that macropores in the dry state of compacted Na-montmorillonite are filled with gel phases, and the grain sizes of clay particles shifted toward smaller values through the saturation and swelling processes. By contrast, no gel phase and no decrease in the grain and pore volumes were observed for saturated Cs-montmorillonite. The geometrical factors of the macropores including tortuosity and geometric constrictivity of saturated Cs-montmorillonite determined by the X-ray CT was consistent with the corresponding values derived in the HDO diffusion test. In the case of Na-montmorillonite, the larger differences between the geometric factors evaluated by the X-ray CT and the diffusion tests can be explained by the electrostatic constrictivity factor and the additional geometrical factors in gel phase and interlayer that are smaller than the detection limit of the X-ray CT.
Yotsuji, Kenji*; Tachi, Yukio; Kawamura, Katsuyuki*; Arima, Tatsumi*; Sakuma, Hiroshi*
Nendo Kagaku, 58(1), p.8 - 25, 2019/00
Molecular dynamics (MD) simulations were conducted to investigate physical properties of water and cations in montmorillonite interlayer nanopores. The swelling behaviors and hydration states were firstly evaluated as functions of interlayer cations and layer charge. The diffusion coefficients of water and cations in interlayer nanopores were decreased in comparison with those in bulk water and came closer to those in bulk water when basal spacing increased. The viscosity coefficients of interlayer water estimated indicated a significant effect of viscoelectricity at 1- and 2-layer hydration states and higher layer charge of montmorillonite. These trends from MD calculations were confirmed to be consistent with existing measured data and previous MD simulation. In addition, model and parameter related to viscoelectric effect used in the diffusion model was refined based on comparative discussion between MD simulations and measurements. The series of MD calculations could provide atomic level understanding for the developments and improvements of the diffusion model for compacted montmorillonite.
Arima, Tatsumi*; Idemitsu, Kazuya*; Inagaki, Yaohiro*; Kawamura, Katsuyuki*; Tachi, Yukio; Yotsuji, Kenji
Progress in Nuclear Energy, 92, p.286 - 297, 2016/09
Times Cited Count:11 Percentile:68.36(Nuclear Science & Technology)Diffusion and adsorption behavior of uranyl (UO ) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO
) species is important for the performance assessment of radioactive waste disposal. The diffusion behaviors of UO , K
, K , CO
, CO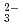 and Cl
and Cl and H
and H O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO
O in the aqueous solutions were evaluated by molecular dynamics (MD) calculations. The diffusion coefficient (De) of UO is the smallest and is 26% less than the self-diffusion coefficient of H
is the smallest and is 26% less than the self-diffusion coefficient of H O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO
O. For the aqueous solution with high concentration of carbonate ions, uranyl carbonate complexes: UO CO
CO and UO
and UO (CO
(CO )
) can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO
can be observed. For the clay (montmorillonite or illite)-aqueous solution systems, the adsorption and diffusion behaviors of UO and K
and K were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO
were evaluated by MD calculations. The distribution coefficients (Kd) increase with the layer charge of clay, and Kd of UO might be smaller than that of K
might be smaller than that of K . Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
. Further, their two-dimensional diffusion coefficients were relatively small in the adsorption layer and were extremely small for illite with higher layer charge.
 activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditions
activity and temperature on the dissolution rate of compacted montmorillonite under highly alkaline conditionsSawaguchi, Takuma; Tsukada, Manabu; Yamaguchi, Tetsuji; Mukai, Masayuki
Clay Minerals, 51(2), p.267 - 278, 2016/05
Times Cited Count:7 Percentile:24.47(Chemistry, Physical)The dependences of the dissolution rate of compacted montmorillonite on activity of OH (a
(a -) and temperature (T) were investigated. The dissolution rate of montmorillonite (
-) and temperature (T) were investigated. The dissolution rate of montmorillonite (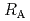 ) in compacted pure montmorillonite, which was formulized as
) in compacted pure montmorillonite, which was formulized as 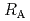 = 10
= 10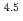 (a
(a -)
-)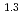 e
e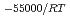 , was higher than that in the compacted sand-bentonite mixtures:
, was higher than that in the compacted sand-bentonite mixtures: 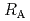 = 3500 (a
= 3500 (a -)
-)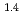 e
e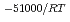 . The difference can be explained by considering the decrease in a
. The difference can be explained by considering the decrease in a - in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a
- in the mixtures accompanied by dissolution of accessory minerals such as quartz and chalcedony. The dissolution rate model developed for pure montmorillonite is expected to be applied to bentonite mixtures if quantification of the decrease in a - is achieved somehow.
- is achieved somehow.
Okubo, Takahiro*; Ibaraki, Moe*; Tachi, Yukio; Iwadate, Yasuhiko*
Applied Clay Science, 123, p.148 - 155, 2016/04
Times Cited Count:29 Percentile:74.44(Chemistry, Physical)The pore distribution of water-saturated compacted clay (Na-montmorillonite at 0.8 and 1.4 g/cm saturated by three salt concentrations) was evaluated using
saturated by three salt concentrations) was evaluated using  H NMR relaxometry and freezing point depression. The populations of interlayer water with four hydrated state and non-interlayer water were calculated from the assumed thresholds. The sample with lower density exhibits higher population of non-interlayer water up to 55%. Low-temperature
H NMR relaxometry and freezing point depression. The populations of interlayer water with four hydrated state and non-interlayer water were calculated from the assumed thresholds. The sample with lower density exhibits higher population of non-interlayer water up to 55%. Low-temperature  H NMR experiments in view of freezing point depression indicated that mesopore water in approximately 4 nm space observed in the calorimetric study was considered as non-interlayer water and the threshold temperature. The result showed that population of non-interlayer water by expected from freezing point depression agreed with
H NMR experiments in view of freezing point depression indicated that mesopore water in approximately 4 nm space observed in the calorimetric study was considered as non-interlayer water and the threshold temperature. The result showed that population of non-interlayer water by expected from freezing point depression agreed with  H NMR relaxometry within 10%. Correlation experiments between longitudinal (
H NMR relaxometry within 10%. Correlation experiments between longitudinal (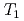 ) and transverse relation times (
) and transverse relation times (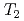 ) at -10
) at -10 C suggested that high-mobility bulk-like water molecules existed at a clay density of 1.4 g/cm
C suggested that high-mobility bulk-like water molecules existed at a clay density of 1.4 g/cm .
.
 on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modeling
on Na-montmorillonite studied by time-resolved laser fluorescence spectroscopy and surface complexation modelingSasaki, Takayuki*; Ueda, Kenyo*; Saito, Takumi; Aoyagi, Noboru; Kobayashi, Taishi*; Takagi, Ikuji*; Kimura, Takaumi; Tachi, Yukio
Journal of Nuclear Science and Technology, 53(4), p.592 - 601, 2016/04
Times Cited Count:12 Percentile:74.83(Nuclear Science & Technology)The influences of pH and the concentrations of Eu and NaNO
and NaNO on the sorption of Eu
on the sorption of Eu to Na-montmorillonite were investigated through batch sorption measurements and time-resolved laser fluorescence spectroscopy (TRLFS). The pH had a little effect on the distribution coefficients (Kd) in 0.01 M NaNO
to Na-montmorillonite were investigated through batch sorption measurements and time-resolved laser fluorescence spectroscopy (TRLFS). The pH had a little effect on the distribution coefficients (Kd) in 0.01 M NaNO , whereas the Kd strongly depended on pH at 1 M NaNO
, whereas the Kd strongly depended on pH at 1 M NaNO . A cation exchange model combined with a one-site non-electrostatic surface complexation model was successfully applied to the measured Kd. The TRLFS spectra of Eu
. A cation exchange model combined with a one-site non-electrostatic surface complexation model was successfully applied to the measured Kd. The TRLFS spectra of Eu sorbed were processed by parallel factor analysis (PARAFAC), which corresponded to one outer-sphere (factor A) and two inner-sphere (factor B and C) complexes. It turned out that factors A and B correspond to Eu
sorbed were processed by parallel factor analysis (PARAFAC), which corresponded to one outer-sphere (factor A) and two inner-sphere (factor B and C) complexes. It turned out that factors A and B correspond to Eu sorbed by ion exchange sites and inner-sphere complexation with hydroxyl groups of the edge faces, respectively. Factor C became dominant at relatively high pH and ionic strength and likely correspond to the precipitation of Eu(OH)
sorbed by ion exchange sites and inner-sphere complexation with hydroxyl groups of the edge faces, respectively. Factor C became dominant at relatively high pH and ionic strength and likely correspond to the precipitation of Eu(OH) on the surface.
on the surface.
Tsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru; Suzuki, Shinichi; Yoshii, Kenji; Nishihata, Yasuo; Yaita, Tsuyoshi
Clay Science, 18(4), p.93 - 97, 2014/12
Yamaguchi, Tetsuji; Sakamoto, Yoshifumi; Akai, Masanobu; Takazawa, Mayumi; Iida, Yoshihisa; Tanaka, Tadao; Nakayama, Shinichi
Physics and Chemistry of the Earth, 32(1-7), p.298 - 310, 2007/00
Times Cited Count:43 Percentile:72.99(Geosciences, Multidisciplinary)Dissolution rate of montmorillonite, diffusivity of hydroxide ion and permeability coefficient in compacted sand-bentonite mixtures were experimentally determined and formulated. A coupled mass-transport/chemical-reaction code was developed to predict variation in permeability of engineered bentonite barrier with alkaline fluid by using the formulae.
Kozai, Naofumi; Mitamura, Hisayoshi; Onuki, Toshihiko; Sakai, Takuro; Sato, Takahiro; Oikawa, Masakazu*
Nuclear Instruments and Methods in Physics Research B, 231(1-4), p.530 - 535, 2005/04
Times Cited Count:9 Percentile:55.97(Instruments & Instrumentation)Applicability of micro-PIXE analysis to quantitative evaluation of heavy elements sorbed on minerals was investigated to get better understanding of sorption and distribution of heavy elements onto mixture of minerals in soil. For this, external standards, that is, heavy element-sorbing minerals of uniform shape and size, were analyzed by micro-PIXE. It was found that such external standards were available to quantitative evaluation by micro-PIXE though their applicability may be limited.
Nakayama, Shinichi; Sakamoto, Yoshifumi; Yamaguchi, Tetsuji; Akai, Masanobu; Tanaka, Tadao; Sato, Tsutomu*; Iida, Yoshihisa
Applied Clay Science, 27(1-2), p.53 - 65, 2004/10
Times Cited Count:81 Percentile:89.26(Chemistry, Physical)Alkaline environments induced by cement in radioactive waste repositories are likely to alter montmorillonite, the main constituent of bentonite buffer materials. Over long time periods, the alteration may cause the physical and/or chemical properties of the buffer to deteriorate. For the purpose of acquiring numerical data to quantify the effect of alteration on permeability of bentonite buffer, dissolution rates of montmorillonite and diffusivity of hydroxide ions in compacted sand-bentonite mixture specimens have been measured under highly alkaline, simulated groundwater conditions. The dissolution rate of montmorillonite was given by the linear dependence on time under the employed experimental conditions of pH 13 to 14 and temperatures of 90 to 170 C. The diffusivity of hydroxide ions was obtained in through-diffusion experiments combined with a pore diffusion model. The experiments were performed under relatively low temperatures of 10 to 50
C. The diffusivity of hydroxide ions was obtained in through-diffusion experiments combined with a pore diffusion model. The experiments were performed under relatively low temperatures of 10 to 50 C to minimize the effect of alteration of bentonite. The effective diffusivity was on the order of 10
C to minimize the effect of alteration of bentonite. The effective diffusivity was on the order of 10 to 10
to 10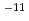 m
m /s.
/s.
Kozai, Naofumi; Adachi, Yoshifusa*; Kawamura, Sachi*; Inada, Koichi*; Kozaki, Tamotsu*; Sato, Seichi*; Ohashi, Hiroshi*; Onuki, Toshihiko; Bamba, Tsunetaka
Journal of Nuclear Science and Technology, 38(12), p.1141 - 1143, 2001/12
This study briefly reports characteristics of Fe-montmorillonite. Fe-montmorillonite was used as a simulant of buffer material in which corrosion products of carbon steel overpack, Fe , were diffused. We have found that this clay retains Se(VI) for which natural montmorillonite, such as Na+-type and Ca
, were diffused. We have found that this clay retains Se(VI) for which natural montmorillonite, such as Na+-type and Ca -type, has little retentivity.
-type, has little retentivity.
Kozai, Naofumi; Inada, Koichi*; Kozaki, Tamotsu*; Sato, Seichi*; Ohashi, Hiroshi*; Bamba, Tsunetaka
Journal of Contaminant Hydrology, 47(2-4), p.149 - 158, 2001/02
Times Cited Count:15 Percentile:41.97(Environmental Sciences)no abstracts in English
Kozai, Naofumi; Onuki, Toshihiko; Muraoka, Susumu
Materials Research Society Symposium Proceedings, Vol.353, 0, p.1021 - 1028, 1995/00
no abstracts in English
Tachi, Yukio; Yotsuji, Kenji; Ito, Tsuyoshi; Suyama, Tadahiro
no journal, ,
The integrated sorption and diffusion (ISD) model was applied for systems coexisting multispecies Sr (divalent cation Sr and neutral SrSO
and neutral SrSO (aq)) in compacted montmorillonite. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in compacted Na-montmorillonite (dry density of 800 kg/m
(aq)) in compacted montmorillonite. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in compacted Na-montmorillonite (dry density of 800 kg/m ) saturated with three types of Na
) saturated with three types of Na SO
SO solutions (0.05, 0.1, 0.5 M) were measured by the trough-diffusion method. The De and Kd values decreased drastically with increasing porewater salinity. The De for multispecies Sr was determined as the harmonic weight-average considering the two species distribution and their log De values, based on comparison with reactive-transport calculations using the PHREEQC. As a result, the De trend could be quantitatively express by the ISD model considering multispecies contributions. The thermodynamic sorption model considering ion exchange reactions could provide reasonable account of Kd trend as functions of salinity.
solutions (0.05, 0.1, 0.5 M) were measured by the trough-diffusion method. The De and Kd values decreased drastically with increasing porewater salinity. The De for multispecies Sr was determined as the harmonic weight-average considering the two species distribution and their log De values, based on comparison with reactive-transport calculations using the PHREEQC. As a result, the De trend could be quantitatively express by the ISD model considering multispecies contributions. The thermodynamic sorption model considering ion exchange reactions could provide reasonable account of Kd trend as functions of salinity.
Okubo, Takahiro*; Ibaraki, Moe*; Tachi, Yukio; Iwadate, Yasuhiko*
no journal, ,
1H NMR relaxometry was applied for investigation of pore structure in compacted saturated montmorillonite with different salt concentration. The samples compacted to dry densities of 0.8 and 1.2 g/cm were saturated with pure water, 0.05, 0.10, 0.50, and 1.0 M NaCl solutions. Population of water in different hydrated layer corresponding to 1-, 2-, and 3 hydrated layer was estimated from longitudinal relaxation distributions. The population of water in 1-, 2-, and 3 hydrated layers changed as a function of NaCl concentration. The threshold to discriminate between inter- and intra-layer water is 3 hydrated layer, which is questionable from existence of 4 hydrated layer revealed by modelling of X-ray profile. The amount of water in 1- and intra-layer were negligible small for all conditions at dry density of 1.2 g/cm
were saturated with pure water, 0.05, 0.10, 0.50, and 1.0 M NaCl solutions. Population of water in different hydrated layer corresponding to 1-, 2-, and 3 hydrated layer was estimated from longitudinal relaxation distributions. The population of water in 1-, 2-, and 3 hydrated layers changed as a function of NaCl concentration. The threshold to discriminate between inter- and intra-layer water is 3 hydrated layer, which is questionable from existence of 4 hydrated layer revealed by modelling of X-ray profile. The amount of water in 1- and intra-layer were negligible small for all conditions at dry density of 1.2 g/cm . On the other hand, water in 2-hydrated layer was decreased instead of increment of water in 3-hydrated layer.
. On the other hand, water in 2-hydrated layer was decreased instead of increment of water in 3-hydrated layer.
Sakuma, Hiroshi*; Tachi, Yukio; Yotsuji, Kenji; Kawamura, Katsuyuki*
no journal, ,
Clay minerals are good adsorbents of many toxic elements of molecules in natural environment, because of their large surface areas and their high affinity to organic and inorganic materials. The adsorption sites of ions and molecules on the basal planes can be estimated by experiments and computer simulations based on the simple surface structure. While the adsorption of ions and molecules on the edge planes of clay minerals is poorly understood due to the absence of established model of the edge structures. In this study, the edge structures of montmorillonite were examined by the first-principles calculations based on the density functional theory. The effect of isomorphous substitution, layer charge, and positions of interlayer cations was evaluated for four different edge planes by calculating the surface energy. The acidity constant of the edges were calculated by an empirical method. We will discuss possible adsorption sites of cations on these edges.